Abstract
Introduction: Recombinant activated factor VII (NovoSeven®RT, Novo Nordisk A/S) is approved for treatment of bleeding and perioperative management in congenital hemophilia with inhibitors (CHwI), acquired hemophilia (AH), congenital factor VII (FVII) deficiency, and Glanzmann thrombasthenia (GT) with refractoriness to platelets. Serious thrombotic events (TEs) have been reported in clinical trials and postmarketing surveillance; however, the incidence of this risk (rate of thrombosis) is considered to be low within labeled indications. While many general risk factors are noted in the prescribing information (PI), the association of reported TEs with certain risk factors noted in the PI has not been established.
Methods: A retrospective safety assessment of clinical trials and registries, used to support licensure and postmarketing surveillance, was performed from which the rate of thrombosis could be calculated in the 4 indicated disorders. Further, all postmarketing TE case reports in the Novo Nordisk safety database (including from registries, spontaneous reports, and the literature) were assessed to identify any of the risk factors listed in the PI and the temporal relationship to NovoSeven®RT use (defined as within 48 h given 2- to 3- hour half-life).
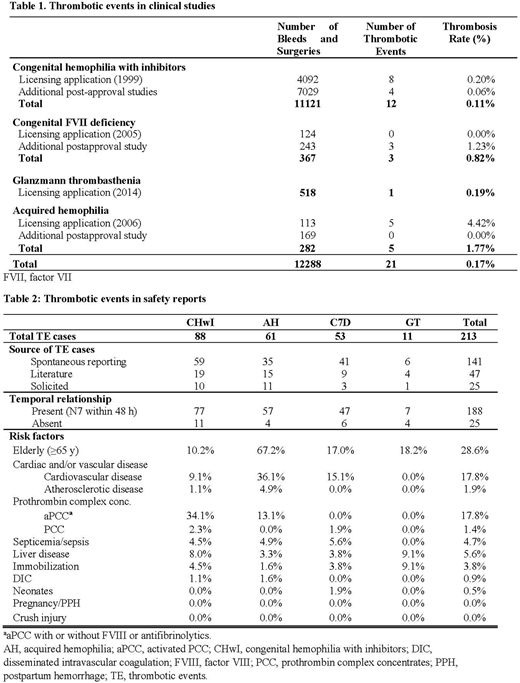
Results: In clinical trials and registries used to support licensure and in postmarketing surveillance, overall rate of thrombosis was 0.17% of 12,288 bleeding and surgical episodes. There were 21 TEs identified (12 CHwI, 5 AH, 3 FVII deficiency, 1 GT). The specific risk by indication (Table 1) was 0.11% for CHwI (11,121 episodes), 0.19% for GT (518 episodes), 0.82% for FVII deficiency (367 episodes) and 1.77% for AH (282 episodes). Analysis of all postmarketing databases (Table 2) revealed 213 TEs (88 CHwI, 61 AH, 53 FVII deficiency, 11 GT). The majority were reported spontaneously (141), with fewer reported in the literature (47) or solicited in registries or investigator-sponsored studies (25). Of 213 TE cases, temporal relationship was assessed as plausible in 188 (88%) if NovoSeven®RT use was reported within 48 hours prior to TE or timing unknown; timing was more than 48 hours in 25 (12%) of cases. Overall, the most common associated risk factor was "elderly," defined in the PI as age ≥65 years (29%), and was particularly common in patients with AH and TE (67%). Another common factor was concomitant use of activated prothrombin complex concentrates (aPCC), identified in 18% of all TEs and 34% of TEs in CHwI. Broadly defined cardiovascular disease (including arrhythmias) was noted in 18% of all TEs (36% AH, 15% FVII deficiency, 9% CHwI) and was much more common than specific note of atherosclerotic disease (2%). Other risk factors listed in the PI were uncommon and, in some cases, never identified in TE reports (eg, postoperative immobilization, sepsis, liver disease, disseminated intravascular coagulation, neonates, pregnancy, postpartum hemorrhage, crush injury).
Conclusions: Data show that the rate of TEs within licensed indications is low (0.17%), as was originally described in the US PI from 1999-2009. It has remained stable over time during postapproval surveillance in multiple US and global registries, with active surveillance for safety information across the 4 approved indications. In postmarketing safety report assessments of patients with CHwI receiving concomitant treatment with aPCCs, older patients, particularly those with AH who are receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing TE.
Rajpurkar:HEMA biologics: Honoraria; Bristol Myers Squibb: Research Funding; Shire: Honoraria; Pfizer: Honoraria, Research Funding; Novonordisk: Honoraria. Croteau:CSL-Behring: Consultancy; Catalyst Biosciences: Consultancy; Genetech: Consultancy, Research Funding; Novo Nordisk: Consultancy; Octapharma: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Spark Therapeutics: Research Funding; Tremeau Pharmaceuticals: Consultancy; Bioveritiv: Consultancy; Biomarin: Consultancy; Bayer: Consultancy; Baxalta/Shire: Consultancy, Research Funding. Boggio:Novo Nordisk: Honoraria. Cooper:Novo Nordisk: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal